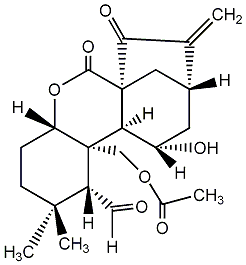
| Name |
Isodonal |
| Formula |
C22H28O7 |
| Mw |
404.18350325 |
| CAS RN |
16964-56-0 |
| C_ID |
C00003443
, 
|
| InChIKey |
QKDLQFSLLCQTOH-WNKPJLINNA-N |
| InChICode |
InChI=1S/C22H28O7/c1-11-13-7-14(25)17-21(8-13,18(11)26)19(27)29-16-5-6-20(3,4)15(9-23)22(16,17)10-28-12(2)24/h9,13-17,25H,1,5-8,10H2,2-4H3/t13-,14+,15-,16+,17-,21+,22+/m1/s1 |
| SMILES |
C1CC([C@H]([C@]2([C@H]1OC(=O)[C@]13[C@H]2[C@H](C[C@H](C1)C(=C)C3=O)O)COC(=O)C)C=O)(C)C |
| Start Substs in Alk. Biosynthesis (Prediction) |
|
| Organism |
| Kingdom |
Family |
Species |
Reference |
| Plantae | Labiatae | Isodon japonica | Ref. |
| Plantae | Labiatae | Isodon japonicus | Ref. |
| Plantae | Labiatae | Isodon rubescens var. lushanensis | Ref. |
| Plantae | Labiatae | Isodon sinuolata | Ref. |
| Plantae | Lamiaceae | Rabdosia macrophylla | Ref. |
|
|
zoom in
| Organism | Isodon japonica |
|---|
| Reference | Ji, et al., Pharmacological Action and Application of Available Antitumor Composition of Traditional Chinese Medicine, Heilongjiang Science and technology Press, Heilongjiang, (1998).
Sun, et al., Brief Handbook of Natural Active Compounds, Medicinal Science and Technology Press of China, Beijing, (1998).
Sun, et al., Diterpenoids from Isodon Species, Science Press, Beijing, (2001).
HAN, et al., Chem Pharm Bull, 51, (2003), 790 |
|---|
|