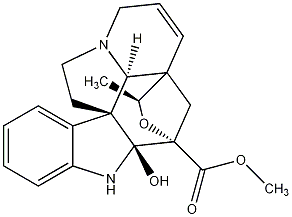
| Name |
Vincoline
20-Epimelobaline |
| Formula |
C21H24N2O4 |
| Mw |
368.17360727 |
| CAS RN |
11034-66-5 |
| C_ID |
C00024612
, 
|
| InChIKey |
YOQNZWXFGROKGY-MVCDMGORNA-N |
| InChICode |
InChI=1S/C21H24N2O4/c1-13-18-8-5-10-23-11-9-19(16(18)23)14-6-3-4-7-15(14)22-21(19,25)20(12-18,27-13)17(24)26-2/h3-8,13,16,22,25H,9-12H2,1-2H3/t13-,16-,18+,19+,20?,21-/m0/s1 |
| SMILES |
c1ccc2c(c1)[C@@]13[C@@](N2)([C@@]2(C[C@@]4([C@@H]1N(CC=C4)CC3)[C@@H](O2)C)C(=O)OC)O |
| Start Substs in Alk. Biosynthesis (Prediction) |
L-Trp Secologanin |
| Organism |
| Kingdom |
Family |
Species |
Reference |
| Plantae | Apocynaceae | Catharanthus roseus  | Ref. |
| Plantae | Apocynaceae | Melodinus morsei | Ref. |
| Plantae | Apocynaceae | Melodinus suaveolens | Ref. |
| Plantae | Rubiaceae | Nauclea officinalis  | Ref. |
|
|
zoom in
| Organism | Catharanthus roseus |
|---|
| Reference | Yin, et al., Modern Study of Chinese Drugs and Clinical Applications (1), Xueyuan Press, Beijing, (1993).
Lin, et al., APS, 24, (1989), 32.
Buckingham(Executive Editor), Dictionary of Natural Products, Chapman & Hall, 1994, Vol1-7
1995, Vol8
1996, Vol9
1997, Vol10
1998, Vol11 |
|---|
|