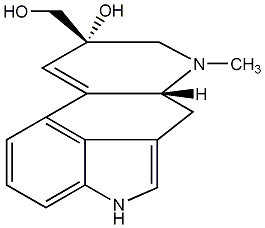
| Name |
Penniclavine |
| Formula |
C16H18N2O2 |
| Mw |
270.13682783 |
| CAS RN |
519-13-1 |
| C_ID |
C00011220
, 
|
| InChIKey |
KCHBNRCSCHMJFD-AQPSWHTGNA-N |
| InChICode |
InChI=1S/C16H18N2O2/c1-18-8-16(20,9-19)6-12-11-3-2-4-13-15(11)10(7-17-13)5-14(12)18/h2-4,6-7,14,17,19-20H,5,8-9H2,1H3/t14-,16+/m1/s1 |
| SMILES |
c1c2c3c(cc1)C1=C[C@](CN([C@@H]1Cc3c[nH]2)C)(CO)O |
| Start Substs in Alk. Biosynthesis (Prediction) |
L-Trp Anthranilate L-Ala IPP |
| Organism |
| Kingdom |
Family |
Species |
Reference |
| Fungi | Clavicipitaceae | Claviceps purpurea  | Ref. |
| Plantae | Convolvulaceae | Argyreia nervosa  | Ref. |
| Plantae | Convolvulaceae | Ipomoea hederacea  | Ref. |
| Plantae | Convolvulaceae | Pharbitis nil  | Ref. |
| Plantae | Poaceae | Pennisetum typhoideum  | Ref. |
| Plantae | Ranunculaceae | Aconitum pendulum | Ref. |
|
|
zoom in
| Organism | Pennisetum typhoideum |
|---|
| Reference | Cole,Handbook of Secondary Fungal Metabolites,Volume I,(2003)
Berde,Handbook of Experimental Pharmacology,Springer-Verlag New York,(1978) |
|---|
|